People’s Choice of Amino Acids: Nominating Our Favorite Protein Building Blocks
written by Kate Jiang
Illustration by Cindy Yimei Wan
The shared carboxyl and amino groups. The single hydrogen that casually hangs on the central carbon. And of course, the varied R-groups that come with at least 20 flavors and give rise to billions of different types of peptides, each with their own functionality; together, they not only potentiated life forms but also made living things mesmerizingly diverse. We have all been there, as young students learning about them for the very first time in the introductory biochemistry course, amazed by their ability to combine their distinct properties together and form the basis of life.
Of all the amino acids that support life, surely one of them must be your “one true a.a.”. Either a particular amino acid’s unique chemistry satisfies your nerdy thirst, or it has some goofy fun fact you simply adore. Driven by curiosity, I went around and asked what everyone’s favorite amino acid is on behalf of Transcripts. Here are some humble qualitative discoveries that I would like to share with you.
Disclaimer: my study is limited by a small sample size, and is heavily biased towards the answers from my colleagues in Transcripts and my poor lab mates…

It All Starts with Methionine
“When asked which amino acid is indispensable, a BCH210 student wrote in an exam: ‘I’d trash methionine, who needs it!’”
–Dr. Reinhart Reithmeier
How can we not start with AUG? As one of the only two amino acids that contain sulfur, methionine plays an essential role in protein synthesis and is always the first amino acid to be added during translation. Truly an irreplaceable amino acid, and certainly not one you would want to trash.
Arginine is Based
“It’s a great solute for stabilizing proteins—at least the L-form is.”
–Anonymous Transcripts member
“[Because of] arginine methylation. My project is literally about it.”
–Anonymous graduate student
“Arginine is assumed to be like lysine—just another positively charged residue—but it is a powerful pi interactor and is great for disordered protein phase separation and dynamic protein interactions in general.”
–Anonymous faculty member
Impressively, the most basic—pKa wise—amino acid received an overwhelming amount of support. While already favored due to its rich interactions through the long and charged side chain, that is not the end of the story: post-translational modifications, roles in phase separation… name it and you shall find it in arginine.
Simplicity is Best—Glycine

“It’s easy to draw—it’s the only thing I know how to draw.”
–Anonymous Transcripts member
“It’s simple and goes through the nanopore nicely [during peptide nanopore sequencing].”
–Anonymous graduate student
“Glycine, because I don’t have to worry about chirality L or D.”
–Anonymous graduate student
Even though it seems to be a “boring” amino acid at a first glance, glycine has in fact gained quite some popularity for its straightforward R-group. Small, flexible, no chirality involved, surely many students will be happy to see it on exams. And don’t forget that it does serve its purpose not only in the cell but also in the lab: imagine running out of glycine when you need to make 10x Tris-Glycine buffer for a gel that you are planning to run this afternoon!
Everybody Loves Aromatics—Phenylalanine, Tyrosine, Tryptophan
“Phenylalanine. Because benzene groups are cool; and although it’s not modified, it is always buried inside the protein and still doing important things in the background.”
–Anonymous lab mate
“Tyrosine is rich in non-covalent interactions.”
–Anonymous Transcripts member
“Tryptophan—because nature liked it so much that they put two rings on it.”
–Average amino acid enjoyer
The three aromatic musketeers nevertheless grabbed a lot of people’s heart. These bulky amino acids add a lot of hydrophobicity and interactions with their sizable rings; in particular, tyrosine has been adored by a lot of people due to its polar hydroxyl group on top of the non-polar interactions. Plus, they also absorb ultraviolet light—which can come in handy for some quick quantifications or quality checks—and serve as precursors for many other biologically relevant small molecules. Of course, things get less fun when you are asked to draw the structure of tryptophan on the whiteboard during your transfer exam.
“His” Aromatic Ring
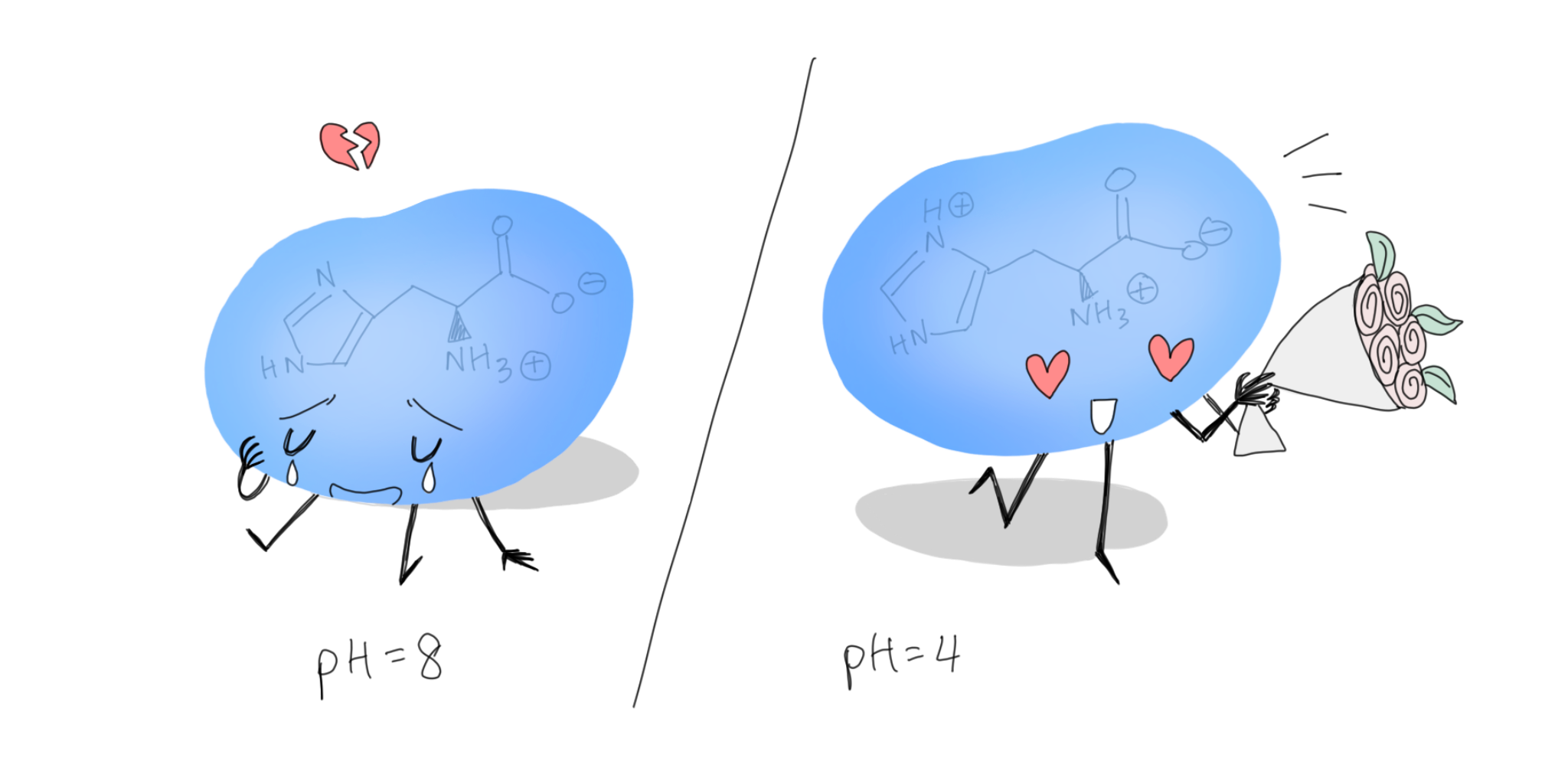
Despite not exactly being an aromatic amino acid, histidine does carry its own imidazole ring and is functionally quite useful. Apart from the rich number of interactions, perhaps the most intriguing thing about histidine is its pKa, which is so close to the physiological pH that its charge shifts depending on the environment. Certainly a cool amino acid to have in the amino acid sequence, and also an indispensable tag for cooler analyses with Ni-NTA column affinity purification.
The Author’s Pick: Serine
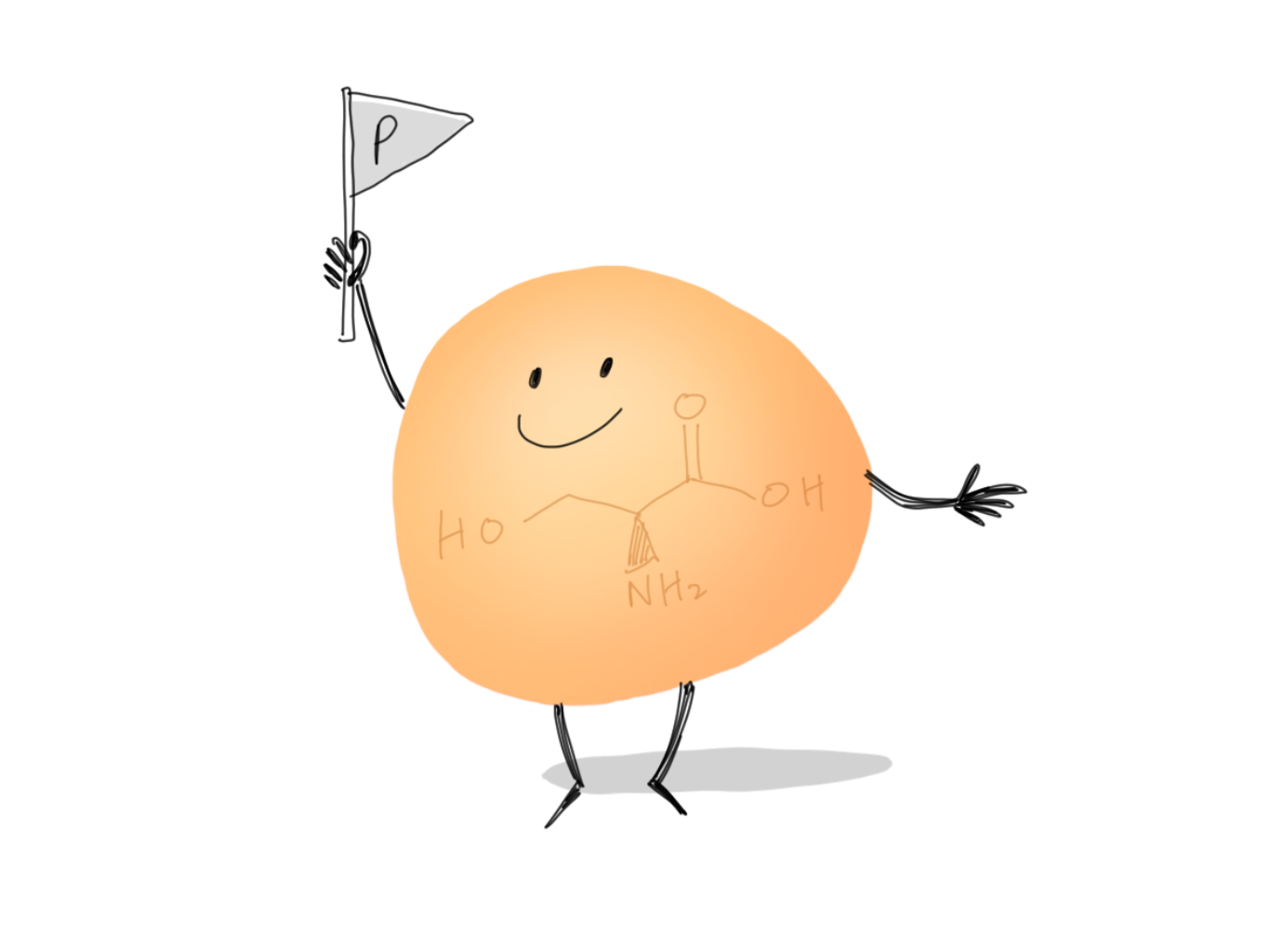
Serine is my personal favorite. A straightforward polar amino acid—who can resist its cute little hydroxyl group? —with a lovely name that reminds me of the word “serene”, it was love at first sight during my introductory biochemistry class. And of course, don’t forget that the most well-known post-translational modification—phosphorylation—can happen on serine! Lots of signaling cascades would be rendered dysfunctional if serine ceased to exist.
However, I should say I am biased towards hydroxyl groups for no particular reason. (Even though the rest of the Transcripts editors did suspect me as an alcoholic when I brought up my love on the OH groups during one of our meetings.)
PRO•LINE
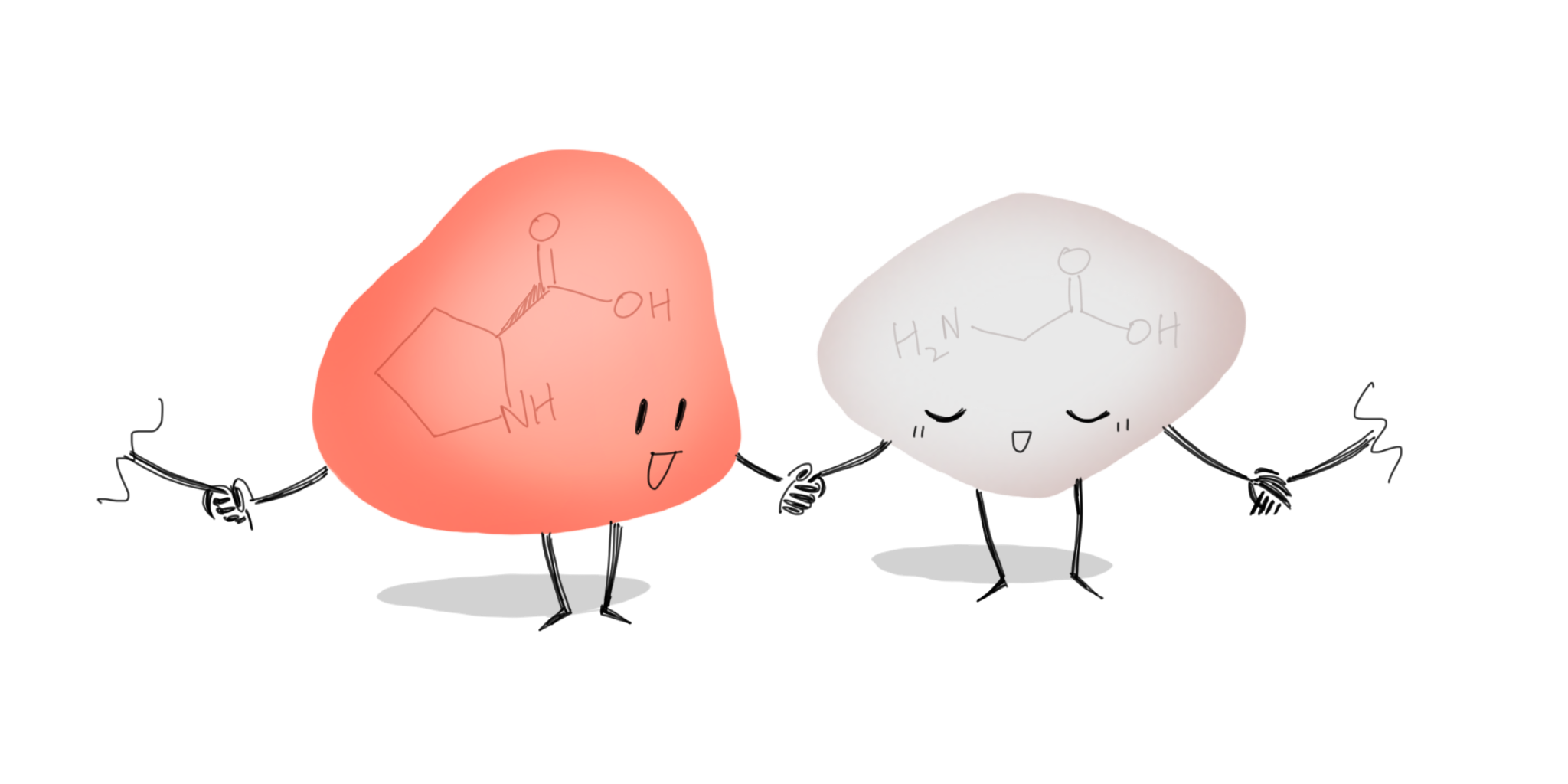
The loopy proline is functionally important in the cell, being the only one that can form a ring with its own amino group nitrogen. In addition, proline can hold glycine’s hand to make beta turns. Yet, this is not why proline was initially nominated; it was the fact that this amino acid shares the same name as a certain gambling game that it drew our attention. (Of course, by no means does Transcripts promote gambling—we just collectively found it funny). Perhaps one day the search engine will return the real proline instead of PROLINE when you type the word in…
