From Cells to Vials, How Biomanufacturing is Done

Written by Annoj Thavalingam
Food, pharmaceuticals, and really anything produced in bulk to enter our bodies, are required by law to adhere to safety and quality regulations dictated by Health Canada. The practices that consistently result in safe-to-consume products can be abbreviated as GMP, or Good Manufacturing Practices. For pharmaceuticals in particular, adhering to GMP involves producing in aseptic environments and following protocols to the T for maximum consistency from one lot to another.
What?
GMP environments, also known as cleanrooms, have a set of controls in place to ensure the working environment is aseptic and as orderly as possible. These controls include:
- Personnel being required to wear gloves, hairnets, shoe covers, sterile suits, and more.
- Frequent circulation of HEPA-filtered air, with air being recirculated up to 60 times per hour per room.
- Floors being mopped several times per week; walls mopped on a monthly basis, and ceilings at least once yearly.
- Regular swabbing of work surfaces, equipment, door handles, and floors to test for the presence of microorganisms.
In a GMP setting, products are generally manufactured in large quantities. By this point in production, protocol optimization has already been performed at lab scale. Hence, procedures carried out at the GMP level are set in stone. Production technicians enter the facility, follow the procedure, and then exit. Unlike in an R&D environment, there is little room for hypothesis building and testing, or wiggle room in how you carry out a procedure. As a result, GMP environments provide greater conformity in the final product’s profile, batch to batch.
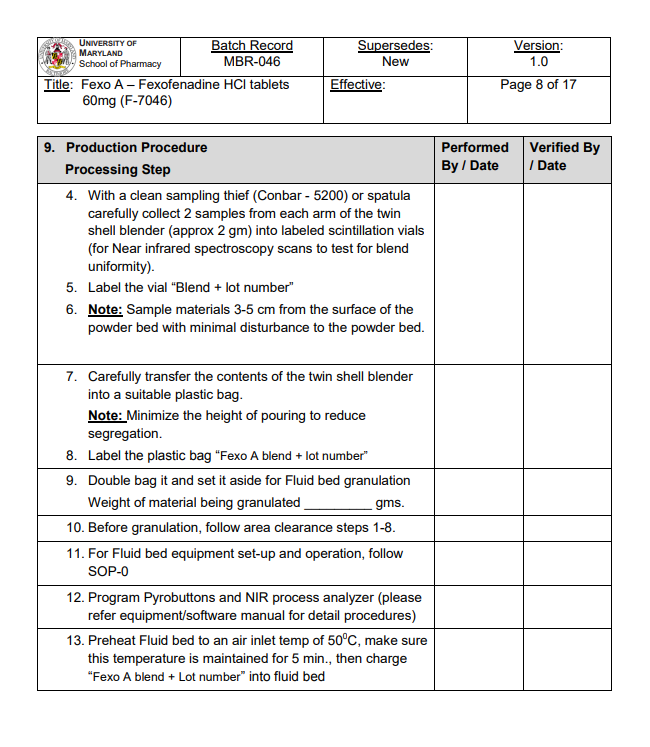
Working in cleanrooms differs from working in research laboratories in several aspects. Firstly, no work can be done alone, no matter how menial. A minimum of two people must be present when performing any task, which ensures accountability in the process. This includes a rigorous documentation process, where the completion of every step in the procedure is dated and signed off by both technicians in real time.
Even the process of setting up at the beginning of your day can take up to an hour or longer. Materials need to be decontaminated before entering the facility, and the lot numbers and expiry dates of all consumables used that day need to be recorded, along with the calibration dates of equipment to be used.
Gowning is also taken very seriously, since you want to limit the spread of microorganisms and cells from your skin and spittle as much as possible. Though requirements may vary from facility to facility, gowning articles generally include: scrubs, gloves, hairnet, safety goggles, beard cover (if applicable), mask, shoe covers and coveralls.

Certain biologics, such as proteins, have been in production for patient treatment for much longer than more novel therapies. Insulin, for example, had been produced in Toronto as early as 1922 to treat patients suffering from Type 1 Diabetes. Although the principles of GMP did not exist at this time, the need to consistently produce compounds of high purity was not lost on researchers. The groundbreaking technology that gave rise to GMP decades later was mass airflow control.
When?
Cleanrooms as we know them today were first invented by Dr. Willis Whitfield, a nuclear physicist working in Albuquerque during the 1960s. At the time, the manufacturing of high-quality nuclear weapon parts was greatly stymied by micron-sized dust particles floating around the lab space. Tasked by his supervisor to resolve the dust issue, Whitfield developed a laminar airflow set-up for the entire production room: air entered from the top of the room and travelled downwards, exiting through grates on the floor. In this way, suspended dust particles are sifted out through the grates and don’t settle on surfaces easily. This single change in airflow reduced dust counts in the air from 1,000,000 particles per ft3 to 750 particles per ft3. And so the cleanroom was born.
The use of cleanrooms today has expanded from nuclear weapons to encompass production of semiconductors as well as (bio)pharmaceuticals.
Who?
Biomanufacturing plants have many different teams that come together to keep the facility afloat. At ground zero, production technicians are in charge of performing activities such as cell culturing or biomolecular purification to produce their biologic(s) of interest. Supporting these activities are several other groups such as Quality Control, Quality Assurance, Validation, Engineering, and Maintenance, to name a few.
Facilities that produce biologics can be classified into one of two categories. The first are original manufacturers, like Sanofi and GSK, who discover and produce their own products in-house. Such manufacturers tend to be big names in the biopharma industry, with the resources to discover biologics and head their own R&D optimizations, scale-up, and mass production. However, most manufacturing facilities are CMOs, or Contract Manufacturing Organizations. As their name suggests, these facilities accept contracts from a variety of companies looking to commercialise and produce biologics at scale. One can expect to learn a greater variety of techniques at CMOs, as they may cater to a broad clientele.
Where?
Over the past few decades, biopharmaceutical production in Canada has been lagging behind the rest of the First World. The COVID-19 pandemic brought this shortcoming to a head, when our government struggled to find partners for in-house vaccine manufacturing. Since 2020, $1.8B in government funds have been allocated across seven provinces for the expansion of current biologics production facilities, as well as the construction of new ones. The table below lists some of the production facilities in Canada, and is by no means exhaustive. Feel free to click on the company names to learn more:
Why?
As biochemistry grad students, many of you may be well-versed in cell culture work and/or biomolecular purification. Although the scale of work may differ in a GMP setting, the science is the same and the techniques similar, making biomanufacturing a viable career option. Depending on what exactly you wish to do day-to-day, a few different departments may be of interest (See Who? above). A point to keep in mind however is that graduate degrees may be overqualified for certain positions. Generally, BSc/MSc degrees are suitable for Production and QC technicians, while managerial positions tend to attract MSc/PhDs (with some initial work experience).
Further Reading
